Experiment with gummy bears
Project created by: Dr Natalie Fey and Dr Ben Mills
The NFWI has teamed up with the University of Bristol to create a series of worksheets for you to try with your WI. What could be sweeter than using gummy bears to uncover the principles of osmosis in this month's experiment?
What’s involved?
Sweets such as gummy bears can be swollen or shrivelled by soaking in water containing salt or sugar. Here we show how you can explore this effect, and understand the principle behind it – osmosis.
You will need:
- Gummy bears (at least 4)
- Sugar (any kind)
- Salt
- Hot water
- Measuring spoons
- 3 cups
- 3 shot glasses
- Kitchen roll
- A plate
- A small stirring stick (perhaps a kebab skewer)
- Pen and paper to make labels
- A suitable work surface for a (stainless steel sink or a glass surface protector; I tend to use my glass hob, which is of course turned off and cold)
Sweets that look similar to gummy bears, such as jelly babies, generally do not work as well for this experiment because they tend to disintegrate more easily. Feel free to try them, but for best results, stick to gummy bears.
You don’t have to use shot glasses: any similar kind of container, such as a cup, which allows you to soak the gummy bears and check how they’re changing, is fine.
Safety
The materials used in this experiment are all cooking ingredients or other edible things, so there should not be much to worry about here beyond the usual kitchen hazards of boiling water and (possibly) broken glass. We do not recommend eating the gummy bears after they’ve been soaked, however, so leave a note to that effect to warn anyone else that comes across your experiment!
Workspace and activity
As we’re using sweets and sugar, this experiment can be a bit sticky. Use easily washed tools and containers, such as those made of glass, plastic or ceramic. Work on a surface that is easily wiped down. Keep an old cloth or kitchen roll to hand to wipe up any spillages.
Procedure
Step 1: Prepare 3 cups: 1 containing 2 teaspoons of sugar, 1 containing a teaspoon of salt, and leaving 1 empty. Label each cup with a paper label, e.g. Su for sugar, Sa for salt and W for water.
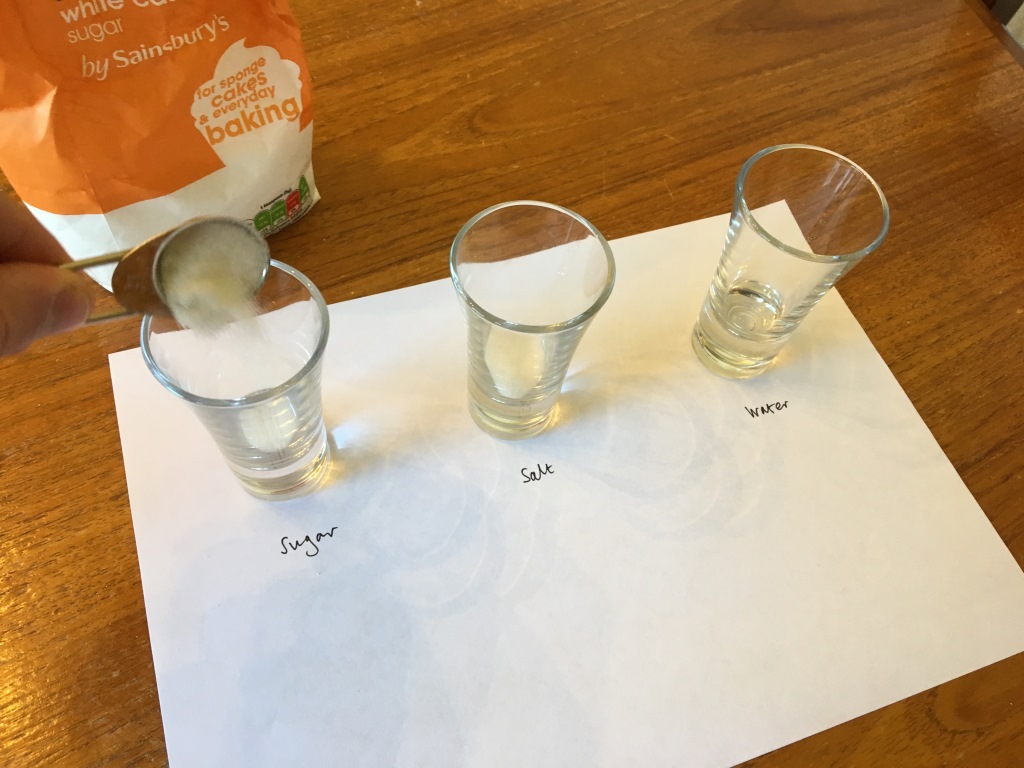
Step 2: Add 20 ml of hot water to each cup and give the sugar and salt glasses a good stir. The hotter the water, the quicker they dissolve, but mind what vessel you use – a cup might be more heat resistant than a shot glass. Leave them to fully dissolve, stirring every so often, if necessary.
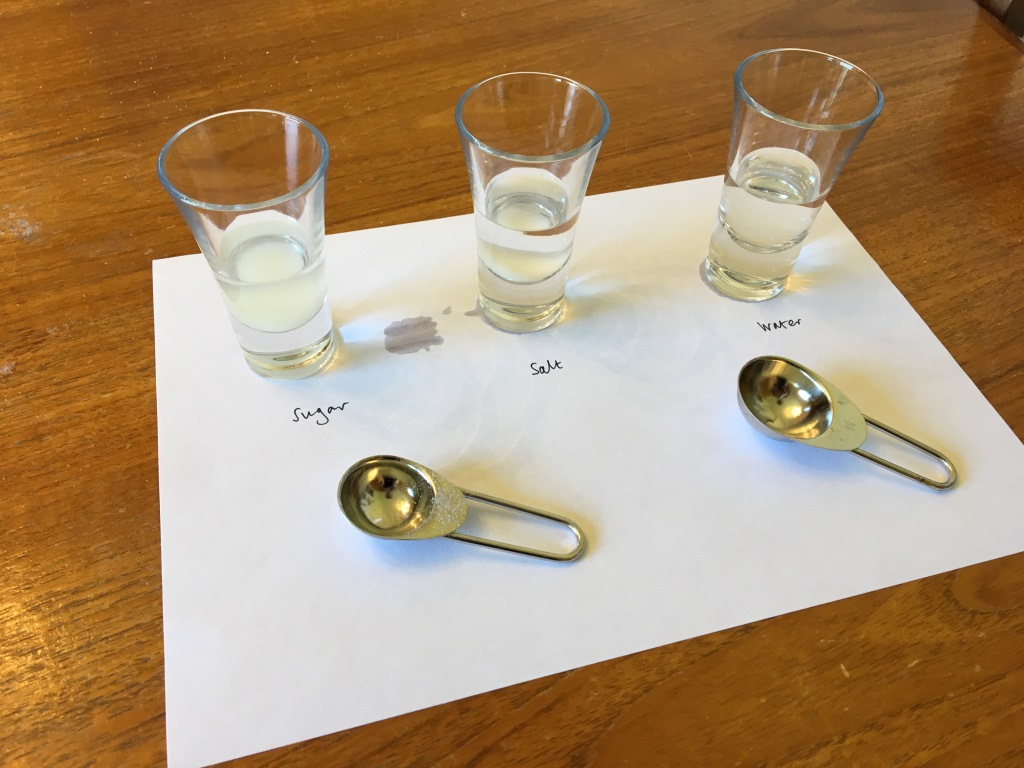
Step 3: Once the sugar and salt has fully dissolved and no white crystals of either substance can be seen in the cups, the solutions are ready. For best results, allow them to cool to room temperature, then pour them into clear shot glasses and drop a gummy bear into each glass. Keep a fourth gummy bear to the side for later comparison with the soaked bears – this is our control experiment.

Step 4: After several hours, you might be able to see differences in shape, size and texture between the soaking bears. When you’re ready to end the experiment, pour off the liquid and collect the gummy bear from each shot glass. Shake any excess liquid off each bear onto some kitchen roll, and then place the bears onto a plate. Compare their size and feel.

Step 5: Wash your solutions down the sink, clean up the workspace and rinse the glassware. Throw away the soaked gummy bears. Hopefully there are some unsoaked bears left in the packet for you after all your hard work.
Brief explanation
If the experiment went to plan, you will have noticed that the sugar-soaked gummy bear swelled to a much larger size than the unsoaked bear, whereas the salt-soaked bear shrivelled to a slightly smaller size. The bear soaked in pure water will have swelled even more than the sugar-soaked bear, but it might have become so large and soft that it disintegrated, or it might have even completely dissolved.
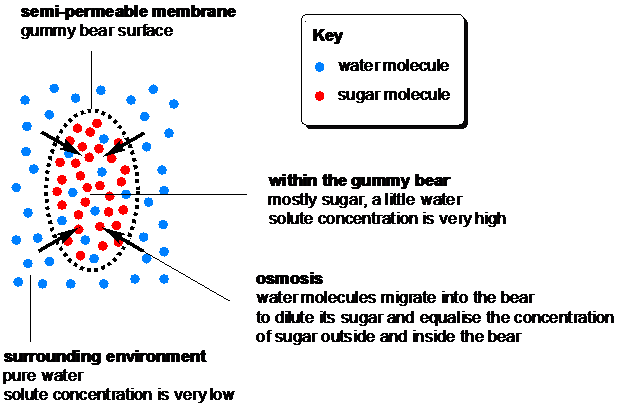
The explanation for these changes is osmosis, the movement of solvent molecules (water in this case) through a semi-permeable membrane (the outer surface of the gummy bear) to a region of higher solute concentration until the solute concentrations either side of the membrane are equal. The semi-permeable membrane is the key to osmosis – it allows only water molecules to pass through, while preventing salt ions or sugar molecules from migrating.
When a gummy bear is soaked in water or a dilute sugar solution, the concentration of solute (sugar and gelatin) is much higher inside the bear than in the surrounding water, so water molecules migrate into the bear to equalise the concentrations and this causes the bear to swell.
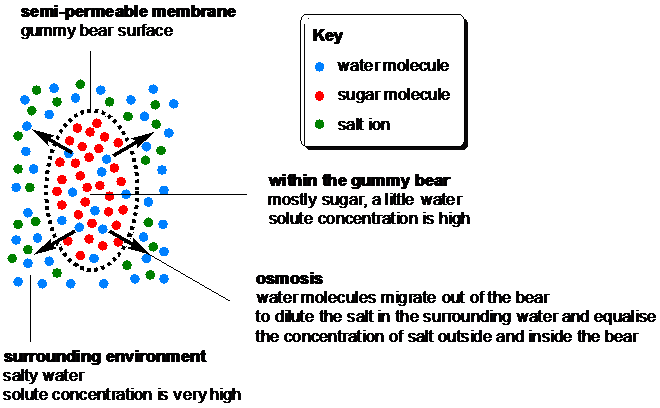
When a gummy bear is soaked in salty water, the concentration of solute (salt) is much higher in the solution outside the bear than inside it, so water molecules migrate out of the bear into the salty water to dilute it and equalise the salt concentration. The movement of water out of the bear causes it to shrivel, although there is only so much water within the bear so there is a limit to how small the bear will get.
Gummy bears’ gelatin bodies retain their shape as water molecules migrate in and out of the bears.
Find more details and a full procedure on the RSC Learn Chemistry website.
