Catalysis with contact lens cleaner and potato
Project created by: Dr Natalie Fey and Dr Ben Mills
The NFWI has teamed up with the University of Bristol to create a series of worksheets for you to try with your WI. This month is an experiment you can do in your kitchen using a few well-known household supplies....
What’s involved?
Using raw potato as a catalyst to get contact lens cleaner to decompose and form bubbles.

You will need:
- A couple of glass vessels – shot glasses or jam jars will work
- A kitchen knife
- A grater
- A suitable work surface – stainless steel sink or a glass surface protector (I tend to use my glass hob, which is of course turned off and cold)
- Washing-up liquid
- Contact lens cleaner which contains hydrogen peroxide (others will not work, but these are usually marked clearly)
- A potato

Safety
You will only use things you are likely to find around the house already or can buy without restrictions, but here are a few tips to help avoid any problems.
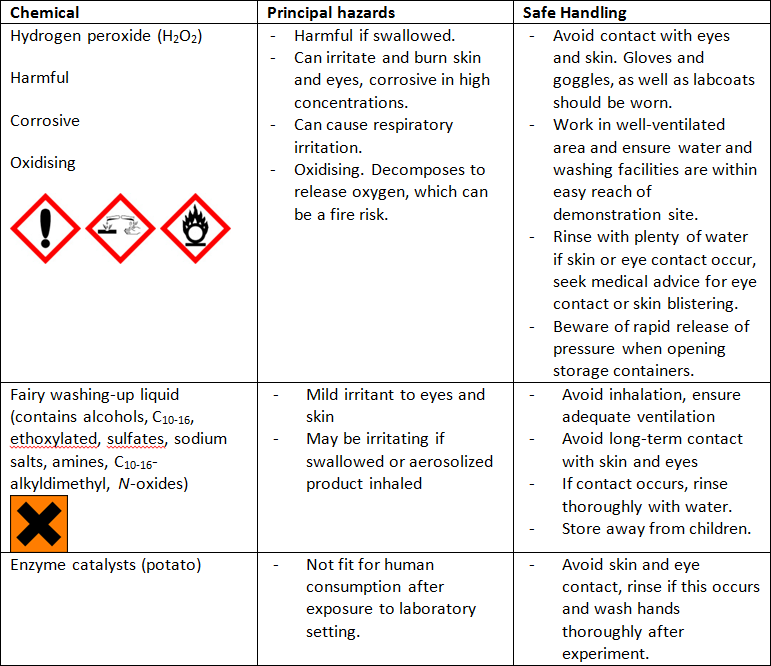
The contact lens cleaning solution contains 3% hydrogen peroxide, which is pretty dilute but could potentially irritate the skin, eyes and respiratory system. Check the safety advice that comes with the cleaning solution you’re using. If you get any of it on your skin or in your eyes, wash the affected area with plenty of water. If it still stings, see a doctor.
Washing-up liquid, perhaps surprisingly, is also irritating to the eyes and even the skin over a long period of time. Wash it off if you experience any irritation.
The potato becomes unfit for human consumption once it has been exposed to the hydrogen peroxide and washing-up liquid, so throw it in the bin after use. The worms in your compost bin won’t like it any more, either.
Ideally, wear gloves and safety specs (often sold in DIY stores), and work on a sturdy surface, but at the very least use small amounts and if you get anything on your skin or face, wash it off straight away.
You can find further details of the potential hazards associated with the substances used in this experiment in the table at the end of the document.
Workspace and activity
Hydrogen peroxide releases oxygen when it decomposes, which can intensify a fire, so keep it away from open flames. The decomposition can also cause a build-up of pressure, so don’t seal the reaction vessel (e.g. jam jar) or leave the contact lens cleaner solution in contact with any catalysts (metals, potatoes, etc.) other than when you intend to (i.e. in steps 3 and 4 in the procedure).
Hydrogen peroxide can bleach fabrics and carpets, so you should work on a suitable surface – a stainless steel sink can be useful in case anything overflows, but a glass surface protector should also work. Avoid working over a carpeted area or on unfinished wood, and have an old cloth or kitchen roll to hand to wipe up any spillage.

Procedure
- Step 1: Put some contact lens cleaner solution into each of two glass vessels. You will not need a lot, but it should cover the bottom and be at least a few millimetres high. If the glass is clean, you should not see any reaction (i.e. bubbles) yet.
- Step 2: Add a bit of washing-up liquid to each of these glasses. This will be more viscous (gloopy, for want of a better description) than the contact lens cleaner, but you should not see any vigorous bubbling.
- Step 3: Cut a small piece off your potato. Carefully drop it into one of the glasses and observe what happens.You should see small trails of bubbles forming all over the cut surfaces (although almost none in areas where there is still potato skin). Over time, you will also see a layer of foam on top of the liquid, where the bubbles have become trapped in the washing up liquid.
- Step 4: Carefully grate a fresh small piece of potato and put the grated potato in the other glass, which already contains contact lens cleaner and washing-up liquid. Again, observe whether there is any reaction and compare the amount of foam formed with the first reaction.You should see a much more vigorous reaction occurring, producing more foam than with the cut potato
- Step 5 (optional): If your contact lens cleaning kit comes with a special ‘neutralising’ lens case, prepare another glass vessel with lens cleaner solution and washing-up liquid and immerse the grey disc of the lens case into this mixture. Once more, observe the reaction.
- Step 6: Wash your solution down the sink with lots of water and clean up after yourself. Throw away the potato pieces.
Brief explanation
As indicated on the packet, the contact lens cleaning solution contains 3 % hydrogen peroxide. The molecular formula of hydrogen peroxide is H2O2, which you might think looks quite a lot like the molecular formula of water (H2O). At room temperature and low concentrations, hydrogen peroxide decomposes very slowly to form water and oxygen. The reaction equation for this process is:
2 H2O2 (aq) → 2 H2O (l) + O2 (g)
At higher concentrations of hydrogen peroxide, this reaction also becomes warm, and we call it exothermic because it releases heat. This indicates that the products (water and oxygen) are more favourable than the starting material (hydrogen peroxide).
You might be wondering why there is any hydrogen peroxide at all, given that it decomposes, albeit slowly when it has no help. The answer is that there is a barrier to the reaction (an activation energy), which slows down the reaction and so prevents complete decomposition.However, in the experiments here the reaction was sped up when potato was added.
Potato (along with celery, liver, yeast and many other foods and living organisms) contains an enzyme called catalase, which catalyses the decomposition of hydrogen peroxide (quite damaging to cells and organism) to water and oxygen, allowing this reaction to occur more easily (faster and at lower temperatures).
Catalysis is important to many areas of chemistry, as it changes the speed of a reaction (reaction rate). This is achieved by following a different reaction pathway, which has a lower activation energy, a bit like taking a short-cut around a mountain rather than climbing all the way up and over the top of it. In addition, the catalyst is not used up by the reaction and left unchanged, so it can be used many times, or, in another way of thinking about this, you do not have to add very much.
Catalysts can be destroyed or deactivated by other reactions, though, so even this good thing will not last forever. In addition, where multiple reaction pathways exist in competition, catalysts can be used to favour one possible outcome over many others, making reactions selective. Here, we released a little bit of catalase on the freshly cut surfaces of the potato, while grating it increased the surface area substantially, so more catalase became available to decompose the hydrogen peroxide.
While the same weight of fresh potato should contain about the same amount of catalase, I am not sure the cut potato would catch up with respect to the foam produced – it is likely that the surface will become clogged up with inactive catalase, so the fresh one inside the potato piece will not become used up in the same way as when using grated potato.If you have also tried the grey disc at the bottom of the special lens case, it is likely that this was made from a noble metal.
Metal surfaces can also catalyse the decomposition of hydrogen peroxide and this is a useful way of using up any hydrogen peroxide when cleaning your contacts, thus preventing any leftovers from reaching your eyes.
Possible extension
There are many extensions to this experiment, testing peroxide solutions of different concentrations (hydrogen peroxide is also present in some hair dyes and sold to clean wounds), as well as a range of catalysts, such as celery, yeast dissolved in warm water, uncooked liver and even gold jewellery. By repeating the experiment with a range of different possible catalysts, you can compare how vigorous the reaction is and how long it goes on for. You can also explore what happens if you cook the liver or vegetables used.
In addition, you can investigate this reaction in a more quantitative way by measuring a standard amount of hydrogen peroxide solution in a small measuring jug or with a measuring spoon (make sure to clean these thoroughly before using them again in the kitchen) and then observe with a stopwatch how long it takes to produce a given amount of foam – this will allow comparison of different experimental setups (e.g. different concentrations of peroxide and different catalysts). And if you do a Google search for ‘elephant toothpaste’, there will be further ideas online.
In all of this, please heed the safety information given above and supplied with the compounds you are using and be careful when using raw meat. All foodstuffs need to be thrown away, with work surfaces and equipment thoroughly cleaned.
Figure 1: Potential Hazards
Find more details and a full procedure on the RSC Learn Chemistry website.
